1. Allergy hypothesis: Allergies reveal the infections we have. Severe allergies and infections can be matched up: peanut and Staph, bee and T.gondii, daisy and Clostridium, poison ivy and spirochetes. If an infection can be killed by a compound or inhibited by the compound our immune system sees the interaction and we associate all the compounds present with the infection. If an infection makes a pigment from a compound we will react to that too. For example aflatoxin with peanuts could cause staph to counter respond. Our immune system would identify everything there at the scene of the crime as part of the infection.
2. Quorum hypothesis: Infections talk using quorums and these quorums interfere with our body's pathways. ( for example mycobacteria's cGMP causes such things as type 2 diabetes, high cholesterol, and synuclein bodies)
3. Autoimmune cross-targeting hypothesis: The Cross-targeting of simultaneous infections on one tissue, one infection inside and one infections outside, triggers autoimmunity. Antibodies or chemicals/drugs can replace one of the infections. By antibodies I mean even those triggered by vaccines. For example a flu virus inside of the pancreas and e.coli latching on to the outside could trigger type one diabetes.
4. Co-carcinogenesis: a virus and a carcinogen together cause cancer. Taking Francis Peyton Rous’ hypothesis further, the carcinogen is a polymerase inhibitor which binds the viral polymerase with higher affinity. Thus the DNA methylation state has been changed but the virus is no longer viable. DNA damage does not cause most cancers, the interaction of carcinogens inhibiting viruses do. Further these cancers wear the receptors that the virus used to enter and the characteristics of the cancer will be from the receptor's pathways.
5. The Gluten hypothesis: Barrier crossing infections are ones that can cross the intestine or the blood brain barrier causing gluten sensitivity with the hole they leave behind. This explains the gluten sensitivity of Schizophernia from T.gondii, Celiac disease from e.coli, Tourettes from staph, and crohn’s from mycobacteria.
6. Viral families use receptor families. Influenzas use dopamine receptors. HPVs use Cannabinoid receptors. Herpes viruses use estrogen and estrogen-like receptors. Flaviviruses use melanocortin receptors. Polyomaviruses use serotonin receptors. (note that this would suggest that the ZIKA virus is using the ACTH receptor )
7. Aflatoxin like compounds cause tau bodies. ALS and picks disease are caused by different infections but both have tau. Both infections secrete an aflatoxin like compound. Vitamin D is protective against it because aflatoxin uses the vit D receptor.
8. Pituitary tumors are caused by an infection releasing butyrolactones which interfere with normal GABA signals and cause the immune system to confuse nerves with the infection. (or someone taking too many GHB sleeping pills)
9. The receptors used by a virus when triggering autoimmunity cause pathways to be activated which makes the various types of one autoimmune disease. There are 3 types of schizophrenia which match up with the 3 receptors use by the 3 herpes viruses. (sort of a continuation of 6)
The receptor pathway triggered by a virus involved in an autoimmune disease can cause symptoms of the disease that will be distinct. Example: The 3 types of schizophrenia has all 3 herpes virus families and each receptor activated can be matched to symptoms. Estrogen-related receptor and disorganized symptoms for example. 3 receptors used equals 3 types of schizophrenia
10. Alzheimer's disease is caused by damage to the mitochondria: alpha-herpes family, diacetyl, inherited and linked to down syndrome, or radiation damaged mitochondria.
11. Postural orthostatic tachycardia could be caused by reoviruses and the adrenergic receptors they bind.
12. The HLAs are mailboxes for T cells or NK cells from different areas of the cell. HLA-A is the nuclear mailbox for Tc cells. HLA-B is the mitochondria's mailbox for Tc cells. HLA-DR/DQ is the cytosol's mailbox for RNA viruses for Th cells. HLA-C is the endoplasmic reticulum's mailbox for NK cells. HLA-E is the Golgi, HLA-F is the lysosomes, and HLA-G is the exosomes which also trigger NK cells.
HLA-DQ could be the cytosol's mailbox for non-encapsulated viruses there like reoviruses. HLA-DP is the outer infection mailbox?
The TLR 7 / 9 butterfly nets for DNA in the mitochondria and nucleus trigger TGF-B1 which causes B cells to express MHC1 which are the HLA-A and HLA-B.
HLA-DQ could be the cytosol's mailbox for non-encapsulated viruses there like reoviruses. HLA-DP is the outer infection mailbox?
The TLR 7 / 9 butterfly nets for DNA in the mitochondria and nucleus trigger TGF-B1 which causes B cells to express MHC1 which are the HLA-A and HLA-B.
13. Carcinogens use certain receptors to get into cells which matches them up with specific cancers.
14. TLRs, toll like receptors, are the innane immune system of nets catching conserved molecules in a non specific way but identifying the region as well as general type of infection. Like HLAs they exist in the different areas of the cell. When the internal viral seeking TLRs are activated the IFN matches the region they are in thus the appropriate HLA is produced helping to find the exact culprit.
15. TLRs send IFNs which tells infected cells to express the corresponding HLAs and macrophages to wear the corresponding TAMs (hands to grab infected cells) TAMS: tyro T , axl A , mer M
Nucleus/ Mito TLR7/9 IFNalpha HLA-A HLA-B Mer
Endoplasmic R TLR 8 IFNgamma or more? HLA-C Axl
Golgi TLR3 IFN lamda HLA-D Tyro3
( Cytosol TLR4 IFNbeta which is not an endosome tlr and carried out by Fibroblasts for bacterial infections that enter host cells ? )
Golgi TLR3 IFN lamda HLA-D Tyro3
( Cytosol TLR4 IFNbeta which is not an endosome tlr and carried out by Fibroblasts for bacterial infections that enter host cells ? )
Because an infected ER means little gets to the surface of a host cell the Natural killer cells step in here and secrete IFNgamma. (note that low levels of IFN gamma is secreted to favor MHC2)
Cytosolic viruses go through the golgi before exiting which is why they trigger TLR3.
Cytosolic viruses go through the golgi before exiting which is why they trigger TLR3.
16. More for the Co-carcinogenesis: Nuclear viruses awaken embryonic Hervs. They do so by methylating or demethylating DNA. If the virus family demethylates the cancer will be aggressive while the methylating viruses are slow growing. (Cancer occurs when a carcinogen inhibits the viral polymerase..so the virus opens the DNA up but can't use it)
Note that the infant/child form of genetically caused cancer are for the genes that regulate these embryonic division cycles.
17. Bacterial infections will hide inside of host cells like viruses. Hypothesis: Like the IFN cytokines there should be cytokines that signify where in the host cell they are hiding. Mycoplasmas nest inside the ER, chlamydia hide in vacuoles, salmonella hide in the golgi, and mycobacteria sit in the cytosol. The cytokines seem to match up the features of an infection family with where they hide inside.
For example infections that use modulins tend to move into vacuoles when infecting the host so TLR2 triggers il-23 which then (in hypothesis 20) triggers TGF3 which repairs vacuoles.
18. Could there be 4 different types of asthma caused by different infections which could be diagnosed using cytokine patterns and the allergies present in the host.
19. The problem concerning autism and vaccines could be solved if autism is viewed as an autoimmune disease. The autoimmune cross-targeting hypothesis when applied to autism reveals there could be 3 different types of autism:
Group Flu and RA : frontal lobe autism
Group DTP (tetanus) and HHV6 : temporal lobe autism
Group MMR and sutterella/ campylobacteria: cerebellum autism
Two of these have been linked to vaccines the MMR and the DTP.
Combining the notion that viruses use receptors to enter cells with the notion that "parts" of a virus can set off autoimmunity on the tissue one can only conclude that what is contaminating vaccines setting off autoimmunity are the tiny pieces the virus uses to bind the receptors. Running vaccine solutions through binding columns of the receptors could "clean " the vaccine of the one piece that triggers the autoimmunity.
20. TGF-Beta has been linked to both embryogenesis and cancer as well as the immune system. The area an infection could be damaging causes a specific TGF-B to repair the area. First the TLR butterfly net is triggered by the infection.
TLR9 or TLR7 trigger TGF-B1 which stimulates the mitochondria to grow and smad to the nucleus for DNA repair.
TLR2 triggers TGF-b3 because the infections that use vacuoles tend to be the same ones that use modulins,
TLR-6 which binds lipoproteins found on gram positive bacteria and mycobacterias which divide in the cytosol triggers TGF-b2 which encourages Tcells to check the cytosol of cells,
finally TL3 of the golgi triggers TGf-B4. TGF-B4 stimulates growth of the golgi.
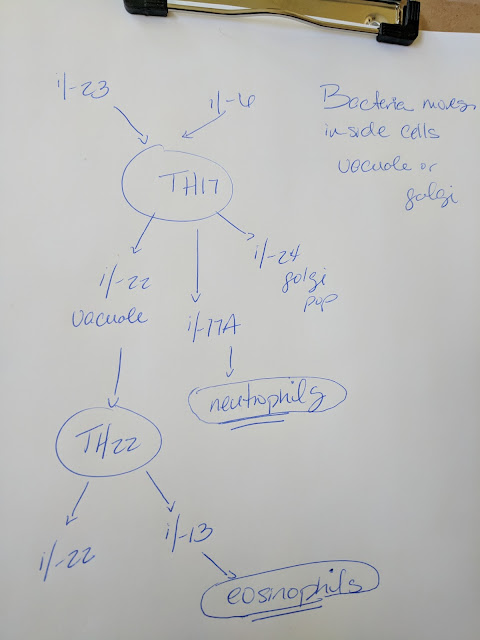
21. T h17 cells are involved in second pops. When does this happen? When large infections move inside of host cells or viral infections are hiding inside of the mitochondria or nucleus. When the viruses are not visible in the cytosol or ER which would be visible when the host cell is popped. Thus a "second popping" must occur.
il-6 and il-23 trigger Th17 to release il-22, il-24, il-17A
note that il-6 is a "reset all in immune respose where phagocytes and TH1 are triggered"and il-23 are released by phagocytes when they can't find the infection they are looking for
il-17a increases neutrophils
il-21 and TGF-b1 trigger the release of il-26, il-19, and il-17F
note that TLR7/9 release TGF-b1 and macrophages release il-21 when activated by viruses
il-17F stimulates CTL
22. The type of Lambda IFN matches up with the type of virus going through the golgi. (looks like all viruses go through the golgi before exiting even when they start in different areas)
IFN lambda1 Nuclear virus
IFN lambda2 Mitochondrial virus
IFN lambda 3 cytosolic virus
23. Hypothesis: the TNF family is involved with infections of the immune system itself. Bcells wear TNF-beta(lymphotoxin-alpha) and secrete it at the peyer patches interacting with FDC. This can be seen with viral infections that invade Bcells. Macrophages wear TNF-c (lymphotoxin-beta)when they are infected with bacterias. Macrophages (and other APC) secrete TNF-alpha when infected with either a virus or a bacteria. They key here is that TNF-c receptors on Mast cells switch the focus of the mast cell. They normally secrete cytokines geared at viruses but after lymphotoxin-beta receptor activation they secrete il-6, il-4, and TNF-alpha.
24. The il-20 family hypothesis of cytokines are H+ pores leaking the H+ out of their assigned membranes (extending hypothesis 21) but I don't know...they could be functioning differently but they are "popping" infections somehow.
il-20 plasma membrane?
il-22 vacuoles
il-24 golgi
il-26 nuclear membrane
il-19 mitochondria
These are used by Th17 to pop membranes. The il-20 cytokines could also used for differentiation for example il-19 converting Th1 to Th2.
Thinking of the T17 cells as Th17 and Tc17 helps. TGF-b1 and il-21 which appear with nuclear and mitochondrial viral infections triggers the il-26 and il-19 pores. While il-6 and il-23 appear and trigger Th17 with bacterias that have moved inside of cells.
Note that the infant/child form of genetically caused cancer are for the genes that regulate these embryonic division cycles.
17. Bacterial infections will hide inside of host cells like viruses. Hypothesis: Like the IFN cytokines there should be cytokines that signify where in the host cell they are hiding. Mycoplasmas nest inside the ER, chlamydia hide in vacuoles, salmonella hide in the golgi, and mycobacteria sit in the cytosol. The cytokines seem to match up the features of an infection family with where they hide inside.
For example infections that use modulins tend to move into vacuoles when infecting the host so TLR2 triggers il-23 which then (in hypothesis 20) triggers TGF3 which repairs vacuoles.
18. Could there be 4 different types of asthma caused by different infections which could be diagnosed using cytokine patterns and the allergies present in the host.
19. The problem concerning autism and vaccines could be solved if autism is viewed as an autoimmune disease. The autoimmune cross-targeting hypothesis when applied to autism reveals there could be 3 different types of autism:
Group Flu and RA : frontal lobe autism
Group DTP (tetanus) and HHV6 : temporal lobe autism
Group MMR and sutterella/ campylobacteria: cerebellum autism
Two of these have been linked to vaccines the MMR and the DTP.
Combining the notion that viruses use receptors to enter cells with the notion that "parts" of a virus can set off autoimmunity on the tissue one can only conclude that what is contaminating vaccines setting off autoimmunity are the tiny pieces the virus uses to bind the receptors. Running vaccine solutions through binding columns of the receptors could "clean " the vaccine of the one piece that triggers the autoimmunity.
20. TGF-Beta has been linked to both embryogenesis and cancer as well as the immune system. The area an infection could be damaging causes a specific TGF-B to repair the area. First the TLR butterfly net is triggered by the infection.
TLR9 or TLR7 trigger TGF-B1 which stimulates the mitochondria to grow and smad to the nucleus for DNA repair.
TLR2 triggers TGF-b3 because the infections that use vacuoles tend to be the same ones that use modulins,
TLR-6 which binds lipoproteins found on gram positive bacteria and mycobacterias which divide in the cytosol triggers TGF-b2 which encourages Tcells to check the cytosol of cells,
finally TL3 of the golgi triggers TGf-B4. TGF-B4 stimulates growth of the golgi.
21. T h17 cells are involved in second pops. When does this happen? When large infections move inside of host cells or viral infections are hiding inside of the mitochondria or nucleus. When the viruses are not visible in the cytosol or ER which would be visible when the host cell is popped. Thus a "second popping" must occur.
il-6 and il-23 trigger Th17 to release il-22, il-24, il-17A
note that il-6 is a "reset all in immune respose where phagocytes and TH1 are triggered"and il-23 are released by phagocytes when they can't find the infection they are looking for
il-17a increases neutrophils
il-21 and TGF-b1 trigger the release of il-26, il-19, and il-17F
note that TLR7/9 release TGF-b1 and macrophages release il-21 when activated by viruses
il-17F stimulates CTL
22. The type of Lambda IFN matches up with the type of virus going through the golgi. (looks like all viruses go through the golgi before exiting even when they start in different areas)
IFN lambda1 Nuclear virus
IFN lambda2 Mitochondrial virus
IFN lambda 3 cytosolic virus
23. Hypothesis: the TNF family is involved with infections of the immune system itself. Bcells wear TNF-beta(lymphotoxin-alpha) and secrete it at the peyer patches interacting with FDC. This can be seen with viral infections that invade Bcells. Macrophages wear TNF-c (lymphotoxin-beta)when they are infected with bacterias. Macrophages (and other APC) secrete TNF-alpha when infected with either a virus or a bacteria. They key here is that TNF-c receptors on Mast cells switch the focus of the mast cell. They normally secrete cytokines geared at viruses but after lymphotoxin-beta receptor activation they secrete il-6, il-4, and TNF-alpha.
24. The il-20 family hypothesis of cytokines are H+ pores leaking the H+ out of their assigned membranes (extending hypothesis 21) but I don't know...they could be functioning differently but they are "popping" infections somehow.
il-20 plasma membrane?
il-22 vacuoles
il-24 golgi
il-26 nuclear membrane
il-19 mitochondria
These are used by Th17 to pop membranes. The il-20 cytokines could also used for differentiation for example il-19 converting Th1 to Th2.
Thinking of the T17 cells as Th17 and Tc17 helps. TGF-b1 and il-21 which appear with nuclear and mitochondrial viral infections triggers the il-26 and il-19 pores. While il-6 and il-23 appear and trigger Th17 with bacterias that have moved inside of cells.
25. 25-hydroxycholesterol (25HC ) is secreted by M1 macrophages to help cells infected with cytosolic viral infections.
No evidence yet that 25HC binds RNA. However the Hydroxyl group of the 25HC could attach to the sugar phosphate backbone of RNA....possible? Exosomes are high in 25HC and CD169 has been shown to bind it. SCS and dendritic cells have cd169. SCS is the macrophage at the lymph that takes internal antigens and presents them to the other side.
26. CSR hormone hypothesis of the germinal center. This is a hypothesis that extends upon another researchers' hypothesis. Hormones of the location are involved in the "class switch recombination" CSR of antibodies. The 3 musketeers of hormones: Insulin, Growth Hormone, and Insulin-like Growth Factor dictate which antibody : IgG1, IgA, or IgE are made by B cells interacting with FDC and TFH.
Note that this germinal center hypothesis only involves the Bcell education of it's BCR of visible foreign matter.
27. The 3 adaptive immune attack zones: outside of cells, inside the cytosol of cells, and inside the mitochondria or the nucleus. The germinal center is the B memory and BCR zone while the cortex region is where T cell memory and TCR receptors are key.
The paracortex zone can be split into 2 inside zones.
Inside the cytosol is the TH1 who interacts with APC MHC2 concerning cytosol infections creating IgG2.
Inside the mitochondria and the nucleus is the Tc who looks at cells' MHC1 with internal organelles infected with viruses directly and tells the B cells to make IgG3.
Also note that these cortex zones is where the T cell education and memory matter. T cells are educated for the inside of cells. The LEC lining the lymph releases il-7 which increases the TCRs . The more the TCRs are activated the more likely a T memory cell is created.
Inside the mitochondria and the nucleus is the Tc who looks at cells' MHC1 with internal organelles infected with viruses directly and tells the B cells to make IgG3.
Also note that these cortex zones is where the T cell education and memory matter. T cells are educated for the inside of cells. The LEC lining the lymph releases il-7 which increases the TCRs . The more the TCRs are activated the more likely a T memory cell is created.
The outside zone is where the germinal center's TFH evolves from TH2. The TH2 pathways involve infections that are visible. The Continued activation of the BCR determines the memory B cells.
28. The dendritic cells support the 3 adaptive zones:
The follicular dendritic cell and the lymphatic dendritic cell display antigens for the BCR for the outer zone.
The myeloid dendritic cells hold antigens in MHC2 for the cytosol zone.
The plasmacytoid dendritic cells hold antigens in MHC1 for the nuclear and mitochondria zones.
http://angelabiggs.blogspot.com/2018/05/hypothesis-dendritic-cells-match-up.html
29. T gamma delta cells function like B cells and produce gamma delta antibodies that look like IgG. Previous researchers have postulated that they go through somatic hypermutation and predate B cells' bcr/ B cell antibodies. IgGamma delta binds mostly lipids and these cells are educated in the spleen. (seen with malaria)
30. The dependence of B cell activation on T cells' cd40 or independence of the T cell (BCR activation matches up with the location of the antigen. Antigens from inside the cell, the cytosol or nucleus or mitochondria, are T cell dependent on cd40. Antigens on the outside of the cell activate B cells through the BCR.
31. NKT function as the T cells with the "lipid TCR" with gamma delta T cells to produce Gamma delta antibodies against lipids? possible?
32. Two types of CTL: Cytosol infection CTL with Fas killing and then the mito/nuclear CTL with TCR killing.
32. Two types of CTL: Cytosol infection CTL with Fas killing and then the mito/nuclear CTL with TCR killing.